Unlocking the combined value of Offspring’s innovative pre-clinical solutions with Flagship’s clinical trial data-driven assay solutions. Broomfield, CO, USA and Södertälje, Sweden – January 23, 2024 – Flagship Biosciences, a leader in spatial biology and biomarker...
News
News and Insights from Our Multidisciplinary Team of Experts
Flagship Biosciences Is Honored with a Best Practices Award
We are honored to announce that Flagship Biosciences has earned a Frost & Sullivan 2023 North American Customer Value Leadership Award in the AI-enabled digital pathology solutions market. Following from detailed evaluation of best practices criteria, the award...
Flagship Biosciences and Genomenon Partner to Advance Precision Medicine Development
Genomenon’s genomic landscapes enhance Flagship’s biomarker services and companion diagnostic development solutions
Broomfield, CO and Ann Arbor, MI – February 7, 2023 – Flagship Biosciences, a leader in spatial biology and biomarker analytics services, today announced a strategic partnership…
Flagship Biosciences hires Tom Turi, Ph.D. as CSO
Accomplished industry executive strengthens company’s position as a leader in spatial biology and biomarker analytics.BROOMFIELD, CO - September 27, 2022 — Flagship Biosciences, Inc., a leader in spatial biology and biomarker analytics services, announced the hiring...
Spatial Biology CRO Flagship Biosciences Acquires Pharma Services Business from Interpace Biosciences
Acquisition significantly expands Flagship’s portfolio of biomarker and analytics servicesBroomfield, CO – August 31, 2022 — Flagship Biosciences, Inc., a leader in spatial biology and biomarker analytics services, announced the acquisition of Interpace Pharma...
A Digital Assay to Streamline PD-L1 IHC: Published in Scientific Reports
The journal Scientific Reports recently published a manuscript on the validation of Flagship’s new digital assay, Tissue Insight (TI) 22C3 NSCLC.1 This technology facilitates the otherwise cumbersome process of quantifying programmed cell death ligand 1 (PD-L1)...
Sign up to receive industry and company news, including The Cut Point, Flagship’s quarterly e-newsletter.
Recent Posts
PUBLICATIONS/POSTERS
Digital and Spatial Characterization of PD-L1 Expression and IVD Performance in the Immune Landscape of Head and Neck Squamous Cell Carcinoma: A Multimodal Approach
Abstract: The anti-PD-L1 antibody (22C3) is used as a companion diagnostic for successful checkpoint inhibitor therapy in head and neck squamous cell carcinoma. The positive predictive value of this assay, however, is less than ideal as a high PD-L1 score does not...
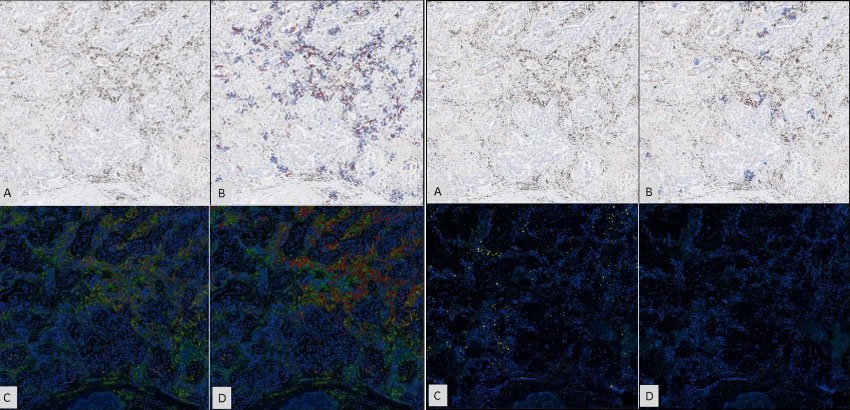
Macrophage Biomarkers CD68 and CD163 Correlate with CRC Patient Survival (Poster No. 2530 at #AACR2022)
Key Takeaways CRC patient survival correlates with the presence or absence of macrophage biomarkers CD68, CD163, and a lack of PD-L1. Lower levels of ‘M1/anti-tumor’ CD68+ or CD68+CD163– cells correlate with increased survival. Higher levels of ‘M2/pro-tumor’ CD163+...
Macrophage Biomarkers CD68 and CD163 Correlate with NSCLC Patient Survival (Poster No. 1720 at #AACR2022)
Key Takeaways Image analysis is able to use immune biomarkers to correlate immune cell presence and localization with patient survival in NSCLC. Patient survival correlates with the presence or absence of CD68 and CD163 macrophage markers, but these markers do not...
EVENTS
2024 Annual Meeting
Meet with us at booth #37085.We help you analyze more data in more depth, so you can produce more effective therapies. Attending the 2024 ASCO® Annual Meeting? Schedule a meeting with our team to discuss our: AI-powered digital pathology solutions Biomarker...
2024 ASGCT Annual Meeting
Are you attending the 2024 ASGCT Annual Meeting?With field-leading keynotes, 200+ scientific sessions, over 7,000 researchers in attendance, and an expanded exhibit hall, the 27th American Society of Gene and Cell Therapy Annual Meeting is the nexus for gene and cell...
AACR Annual Meeting 2024
Meet with us at the AACR Annual Meeting 2024 to learn about our spatial biology solutions. Flagship Biosciences is your source for spatial biology expertise, moving your research forward every step of the way. Our mission is to explore complex biomarkers within the...